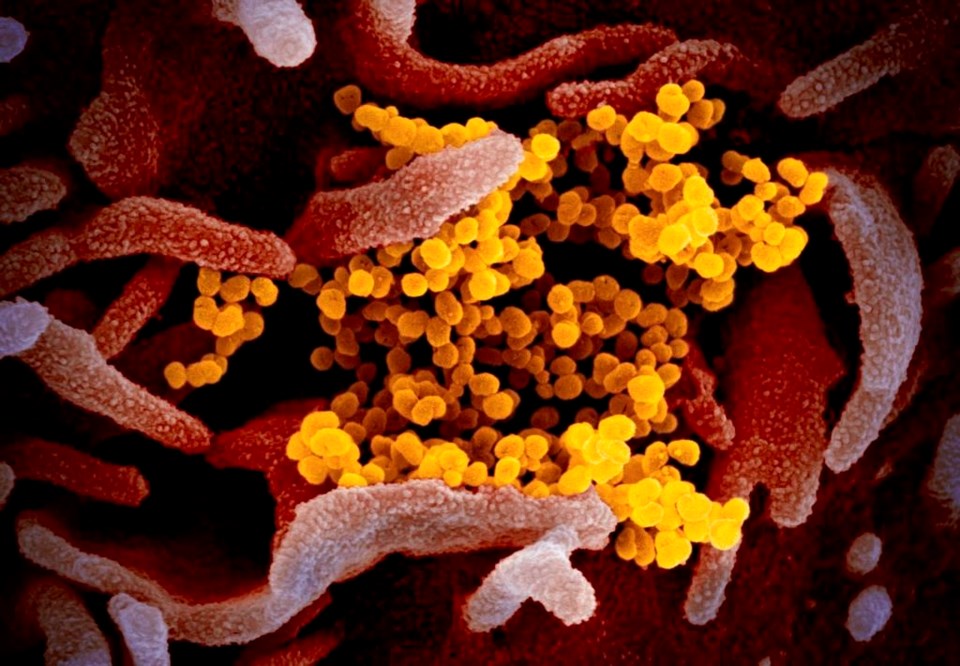
TORONTO — The push to start measuring COVID-19 immunity is gaining steam, with several provinces hinting at plans to roll out blood tests that could pave the way for a return to some semblance of normal everyday life.
So-called serology tests measure the amount of antibodies that appear in the blood after someone has battled an infection — including those who don't even know they had COVID-19 because they didn't meet testing criteria, didn't seek care or had mild or no symptoms.
This data is important for policy makers deciding when to ease restrictions, says epidemiologist Patrick Saunders-Hastings, director of life sciences and environmental health at Gevity Consulting Inc.
Without it, our picture of COVID-19's spread in Canada remains incomplete — diagnostic laboratory tests now in use largely focus on high-risk groups and only capture people actively infected with the virus.
"That's where serological testing comes in," says Saunders-Hastings.
"We just can't — won't — get those counts from the traditional laboratory testing."
Indications various provinces are eager to begin a new phase in detection include ongoing work by the BC Centre for Disease Control to develop a test with the National Microbiology Lab.
Dr. Bonnie Henry, B.C.'s provincial health officer, said Thursday by email that a serology test "should be hopefully coming online in the coming week to ten days."
Meanwhile, Alberta's medical health officer has said her province will nearly triple daily tests in the next month, in part by adding a yet-to-be-approved blood test that reveals who in the community has been exposed to COVID-19.
"Ultimately, our testing goal by mid-to-late May would be approximately 20,000 tests a day. That would be a combination of swab tests as well as serology — the blood tests," Dr. Deena Hinshaw said earlier this week.
A spokesman for Alberta Health adds that the province's public health lab is also working with the National Microbiology Lab to validate multiple serological tests, which would only be used to determine past infection, not current ones.
Saskatchewan's chief medical health officer has said his province, too, had partnered with the Public Health Agency of Canada to investigate serological tests.
Dr. Saqib Shahab predicted Saturday that a serological test "will be available over the next few weeks."
"Over time that will be essential for us to know: How widely has COVID 19 spread? Which age groups is it impacting more? Which age groups and which populations mount the strong immune response?" he said.
Of course the other big question it can help address is: When can physical distancing measures be lifted?
Simply put, a return to normal is contingent on finding as many active and resolved COVID-19 cases as possible.
Those who are immune can help those who are vulnerable, because they won't unknowingly spread the virus, says Saunders-Hastings. They could also return to work.
Estimates of the proportion of asymptomatic infections vary widely, but he says most experts believe it is somewhere between one quarter and half of all cases.
That suggests there could be a significant number of undiagnosed immune people already able to safely return to work, or shoulder more of the frontline health-care burden involving high-risk communities and individuals.
For that reason, effective serological testing shouldn't be bound by the same restrictive criteria being used to conduct DNA diagnostic tests, says Dr. Camille Lemieux, chief of family medicine at the University Health Network in Toronto.
DNA tests are targeted in order to isolate cases and limit COVID-19 spread, but serological tests look for past infections missed or excluded by DNA tests, and that means casting a wide net.
"We need to look more broadly at who has immunity," says Lemieux.
"How many people out there have had it who were really not that sick and either were not tested because they didn't qualify or because they weren't sick enough? Those are the people we need to find and say, 'What percentage of those are immune?'"
Among those waiting for the rollout is the chief financial officer of a Markham, Ont., company with a test waiting for Health Canada approval.
Mitchell Pittaway of BTNX Inc., says his test is already in use by some U.S. labs and is being evaluated in Europe.
"By comparison, most definitely it's slower," he says of Health Canada's approval process, nevertheless believing "they understand the urgency here."
"They're definitely taking this approach which is maybe more structured than what we found with the FDA or in Europe."
If approved, the 15-minute test would be administered by hospitals or clinics, not average Canadians at home, Pittaway says.
He says it's 97 per cent accurate when compared to laboratory PCR methods but is meant to be used in conjunction with a lab test to rule out the possibility a person still has the virus and could infect others.
The results should also be interpreted by a doctor or other health professional to definitively rule out current infection, he adds.
Pittaway says he's been told Health Canada approval partly hinges on the development of a "national strategy."
A co-ordinated coast-to-coast approach is what Lemieux would like to see, and she's urging policymakers to nail that down as soon as possible.
"Now is the time to develop that plan, not in two months when it's like, 'Oh goodness, now we're past the peak, now let's think about our plan.' We need to be proactive," says Lemieux, who also urges Health Canada to accelerate the approval process for new tests.
"I really, really want to see the Public Health Agency of Canada come out with a plan, like a clear plan. And then, the provinces can take their cue from that."
This report by The Canadian Press was first published April 17, 2020.
Cassandra Szklarski, The Canadian Press